Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsHess's Law
The enthalpy change of an reaction is independant for the route undertaken by the reaction as long as the initial and final conditions are the same
I will give a simple example. We can make Sulfuric acid from a simple equation of just SO2 and H2O
But instead we can first react the sulfur dioxide in oxygen then dissolve in sulfuric acid and then dilute it to get the same sulfuric acid as before(conditions must be the same). Theoretically, this should give us the same net enthalpy change for both reactions no matter what route you take
The below diagram is called an Hess's Cycle and you need to know how to draw one. It is very simple and cycle must eventually lead to final products
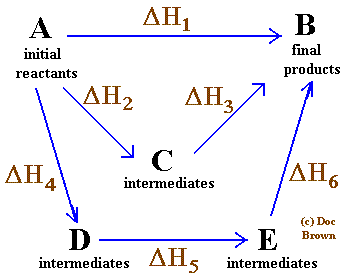
ΔH1 = ΔH2 + ΔH3
Or
ΔH1 = ΔH4 + ΔH5 + ΔH6
Before we get into Hess's law in depth, we will see what is standard enthalpy change of formation
Standard Enthalpy Change of Formation ΔH°f
The enthalpy change when one mole of a compound is formed from its element under standard conditions
This talks about making a single and specific product from its corresponding elements
Na + ½Cl2 → NaCl
A point to remember is that the standard enthalpy change of formation of elements or molecules. For example, ΔH°f[O2] is zero as it is an element. Note that this is only for elements and so it does not apply to CO2 or H2O
Standard enthalpy change of formation can not be found easily as these reactants do not react under standard condition. Because of this we need Hess' Law
Hess' Law in Combustion
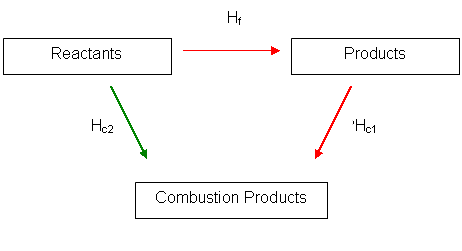
We will take an example. Finding the standard enthalpy change of formation of methane is hard so we must be able to calculate it using Hess' law
Using the combustion details and the value for standard enthalpy change of combustion of methane and the standard enthalpy change of combustion/formation of carbon dioxide and water we can predict the value
Here is the Hess' Cycle
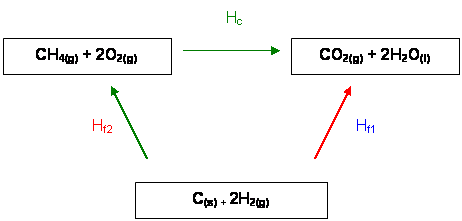
It is clear that:
ΔHf1 = ΔHf2 + ΔHc
As they will give the values of the enthalpy change of formation of water and Carbon dioxide and also the ΔHc. We can find it using this way:
ΔHf1 - ΔHc = ΔHf2
Hess's Law is very difficult to explain without a real example, so we encourage you to look at the video below.
Always make sure you multiply each enthalpy change by the number of atoms needed in the equation. For example if there are 2H2O water molecules then you need to multiply the enthalpy change of formation of water by 2. In fact, I can simplify this into this:
ΔHf1 - ΔHc = ΔHf2
( 2*ΔHf[H2O] + ΔHf[CO2] ) - ΔHc = ΔHf2
These can also be applied to almost to other equations and all
Alternate Method
There is an alternate method from drawing the Hess' cycle and finding the direction and all but this also ultimately uses Hess' law implicitly. We will take the same example of combustion of methane
CH4 + 2O2 → CO2 + 2H2O
C + O2 → CO2 ΔHf[CO2]
H2 + O2 →H2O ΔHf[H2O]
C + 2H2 → CH4 ΔHf[CH4]
So as we can see that hydrogen and carbon dioxide is produced on the right side of the equation
We can combine the equation like we do in half equations to get the full equation. When we combine this it must be the same the one as the original:
CH4 + 2O2 → CO2 + 2H2O
But we need two hydrogen gas and so it must be multiplied by 2
ΔHf[CO2] + 2 * ΔHf[H2O] + ΔHf[CH4] = ΔHc
ΔHf[CO2] + 2 * ΔHf[H2O] -ΔHc = ΔHf[CH4]
This is the same as Hess' law. This is indeed a simple question and for some example it could be harder for example
CO + O2 → CO2
If they have given the ΔHf[CO] then we need to reverse the sign as it is on the left and we need to add the equation together.
C + O2 → CO
CO → C + O2
Also note how we do not include the enthalpy change of formation of Oxygen.
Hess's Law must be mastered by practicing questions and doing MCQ's so we do ask you to practice some past papers
Bond Enthalpy
In your databooklet, they have given the energy required to break one mole of a particular bond. So even using bond enthalpy, we can use it to predict the enthalpy change of a reaction. However, all of the bonds in the system must be identified and their values must be given
ΔH = ∑ Energy bonds broken + ∑ Energy bonds formed
Remember that this considers the sign of the energy for example, bond breaking is absorbing energy so it is + where as bond forming is the energy released which is negative. This could be simplified into this equation if we only consider the size of each energy only
ΔH = ∑ Energy bonds broken - ∑ Energy bonds formed
For example in the databooklet:
The Bond enthalpy of N☰N is around 900. So this is the energy need to break N☰N to form nitrogen atoms
N2 → 2N
This is an endothermic reaction and so the value for all the enthalpies in the databooklet are positive. However, if we need to produce N2 from nitrogen gas. This is the opposite reaction:
2N → N2
This is an exothermic reaction and so this has a value of -900. We can actually simplify it to a Hess' cycle to show what happens during a reaction. The bonds of the reactants are broken and the bonds are formed in the products. The net energy change is the enthalpy change
But to do this we need to draw the structures as it makes it easier to identify which bonds are present. For example:
2C + O2 → 2CO2
C + O=O → O=C=O
O=C=O
So as you can see the energy needed is only needed to break the O=O bond and the energy released when forming bonds are 4* C=O bonds
ΔH = (Energy of O=O) - 4(Energy of C=O)
Substitue the values and find the answer. In either case, the reactants must be broken down to atoms( this is known as the transition state) and those atoms join up to form the product