Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsMoles & Avogadro's Constant
Basics
We will need to know the basic definitions that exams ask
Relative atomic mass - Ar
The average mass of a natural occurring atom of an element on a scale where a carbon atom has exactly 12 units
You can use this definition or the below one
The average mass of a natural occurring atom of an element compared to 1/12 th of a mass of a carbon - 12 atom
A good point to remember is that the mass of 1/12 of C - 12 atom is 1
MCQ's can give it in different ways such as the examples below
R.A.M = Average mass of an atom / ((1/12) * 12)
Same as:
R.A.M = Average mass of an atom *12 / 12
You will need to know how to calculate the relative atomic mass of an atom using different values of Isotopic masses. First let us see what is Isoptopic mass
Isotopes
Isotopes are atoms of the same element which has the same number of protons but different number of neutrons
For example the carbon element has 3 isotopes which are C-12 , C-13 and C-14. These all have the same number of protons because the proton number is used to uniquely identify each element
However, they all have different neutron numbers which results in different mass numbers also ( As mass/nucleon number is the total number of protons and neutrons in the nucleus of an atom)
More of isotopes will be discussed later
Isotopic mass
The mass of an atom of that particular isotope of an element
So you may see that different isotopes have different mass number. The mass number of each atom is called the isotopic mass and it is different for each isotope
Relative Molecular Mass - Mr
It is the mass of a molecule of a substance on a scale where carbon - 12 atom has exactly 12 units
You can also use the other definition if you want
This is found by adding the individual relative atomic masses of atoms in a molecule. For example HCl - 38.5
Relative Formula Mass - Mr
The mass of a formula of a substance on a scale where carbon - 12 atom has exactly 12 units
This is very similar to RMM however we use it in a different situation. We use it only for compounds and giant ionic structures
For example the relative formular mass of NaCl is 58.5
Also remember this is not molar mass but they both have the same values - this is because molar mass has units but RFM has no units
Also the relative formula mass is calculated depending on the formula given - keep in this mind as when the formula simplifies (empirical) it has a different value
Moles
1 mole of any substance has 6.02 * 1023 atoms , molecules or formula units. This is also known as Avogadro's constant
This is how you must be defined
The reason why we use molecules , atoms or formula units is that this depends on the type of structure of the substance.
We will see 3 examples below
Let us take 1 mole of NaCl. This should contain 6 * 1023 of what?
If it was a ionic compound like NaCl then it will give 6 * 1023 formula units or groups of NaCl.
So this means there are 6 * 1023 Na+ ions and also 6 * 1023 Cl- ions
Let's take a different example. Let's see 1 mole of Magnesium
This means that there are 6 * 1023 Mg+ ions in the sample but because it is neutral we can say that it has 6 * 1023 Mg atoms
Lastly, let's see 1 mole of Hydrogen gas
You need to know hydrogen exists as a diatomic moleculeof H2
So 1 mole of H2 will give us 6 * 1023 molecules of hydrogen gas
To find the total number of atoms we just need to multiply by 2 as each molecule has 2 hydrogen atom
Important Equations
Now you need to know the most important equations in this chapter
- Finding the number of moles
- Finding the concentration
- Ioniser and Vaporiser
- Accelerator or an Electric field
- Deflector
- Detector
To find the number of moles just divide the mass of the sample by its molar mass(relative formula mass)
Moles = Mass/Molar Mass
n = m / M
The reason we use molar mass is because it has units of g/mol. So this gives us the correct units for moles
Also remember we use grams as the units for mass
Just divide moles by the number of by the volume of the solution
C = n / V
The units of the concentration is moldm-3
This definition of concentration is very important especialy when talking about how rate is affected by the concentration
Mass Spectrometry
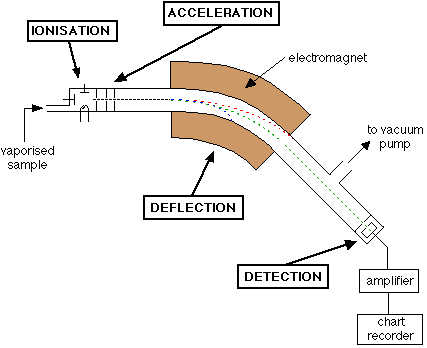
The mass spectrometer is a machine which used to calculate the relative atomic masses of fragments and atoms of different isotopes
We will see how we can use the mass spectrometer to calculate the relative atomic masses by finding the average of the isotopic masses
Before that, we need to know the parts of a mass spectrometer:
This part vaporises the elements to form gaseous atoms which are then bombarded with fast moving electrons. This causes the gaseous atoms to become ionised.
This contains an electric field which accelerates the ions so they will all have the same kinetic energy
The masses will be different so it will result in different speeds
Remember that the work done by an electric field is irrespective of the mass. See more
This is a Tube which contains a magnetic field. This is used to deflect the ions depending on their mass to charge ratio
The Deflection depends on a quantity called mass-charge ratio
If the mass-charge ratio is greater then the deflection is less
Deflection ∝ 1/(mass/charge)
How do you calculate the mass to charge ratio of an ion!
We take the Nucleon number of the ion and divide it by the charge. For example, if I have a Na+ ion which has a nucleon number of 23 then the mass to charge ratio is also 23
23 = 23/1
For most examples and questions, we assume that the charge is +1 only and not greater. This means that the mass to charge ratio is equal to the nucleon number or the isoptic mass of an atom
To summarise, Deflection increases with charge and decreases with mass.
The position of the ions and particles is detected by the detector and is recorded. This depends on the deflection of the ion(mass-charge ratio) but, we assume the charge is always +1
So usually on the detector, ions of the same mass will hit in the same place where as ions of different masses hits in a different place
Usually in exams, they will give an intensity graph where the intensity of each isotopic mass ( same as the mass of the ion ) is given
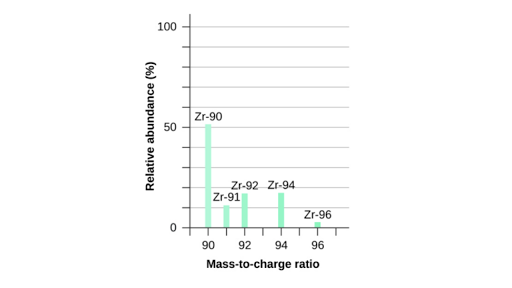
When the intensity is higher and at a greater peak then the relative abudance of the isotope of the element is greater, This means that there is a higher percentage of finding this particular isotope of an element
Here is the equation:
RAM = (Isotopic mass1 * Intensity1 + Isotopic mass2 * Intensity2 +...) / Total Intensity
There maybe many isoptopes for the same element and all will be different
This is gives the average mass of all the isotopes of an element depending on their relative abudance or percentage
The same could be achieved using percentages
We will use an example for this
There are two isoptopes of Sodium. Na - 23 has a relative abudance of 98% whereas Na- 24 has an abundance of 2%. Find the relative atomic mass of Sodium
RAM = (23 * 98 + 24 * 2 ) / 100
RAM = 23.02
Focus on where 98 and 2 adds up to 100. This is divided by 100
You can do it this way also which is basically the same thing
RAM = 23 * 98% + 24 * 2%
In the next chapter, we will discuss what is empirical and molecular formula and how we calculate them