Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsOrganic Chemistry
This is an introduction to organic chemistry. In these series of chapters we will see the different types of organic compounds and the special type of reactions
Hydrocarbon
A Hydrocarbon is a compound that contains only carbon and hydrogen atoms
Good examples are alkenes and alkanes. Alcohols and carboxylic acids are not hydrocarbons
Representations of Organic Compound
There are 5 different representations of organic compounds you need to know and for each we will give an example:
The actual whole number ratio of atoms and elements in a formula of a compound
For example, the Hydrogen peroxide molecule has exactly 2 hydrogens and 2 oxygens
The Molecular formula is:
H2O2
This shows the actual number of atoms present
This is the common way we use to represent organic compounds
It is the simplest whole number ratio of atoms and elements in a formula of a compound
We will use hydrogen peroxide again
The 2 is common for both hydrogen and oxygen so we can divide each by 2
This will give us HO instead of H2O2
This gives us another obvious equation
Molecular formula = n(Empirical formula)
This shows that n number of empirical formulas can fit inside the molecular formula
For more details, go to this chapter
A diagram or drawing of all the bonds in the compound. For example:
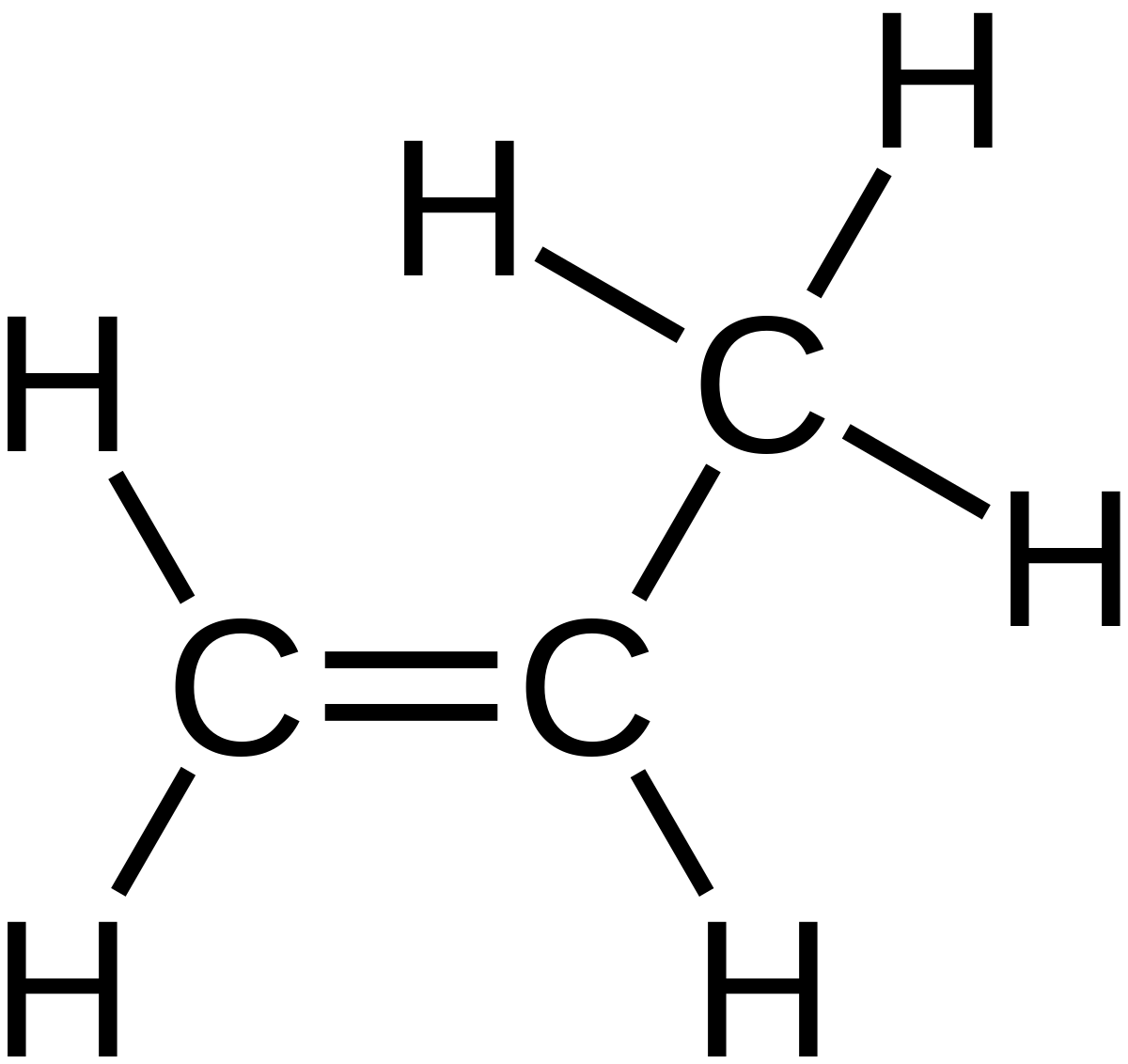
This is similar to the molecular formula but breaks each CH2 or CH3 branches and shows them seperately. It also shows if there is a double bond is present
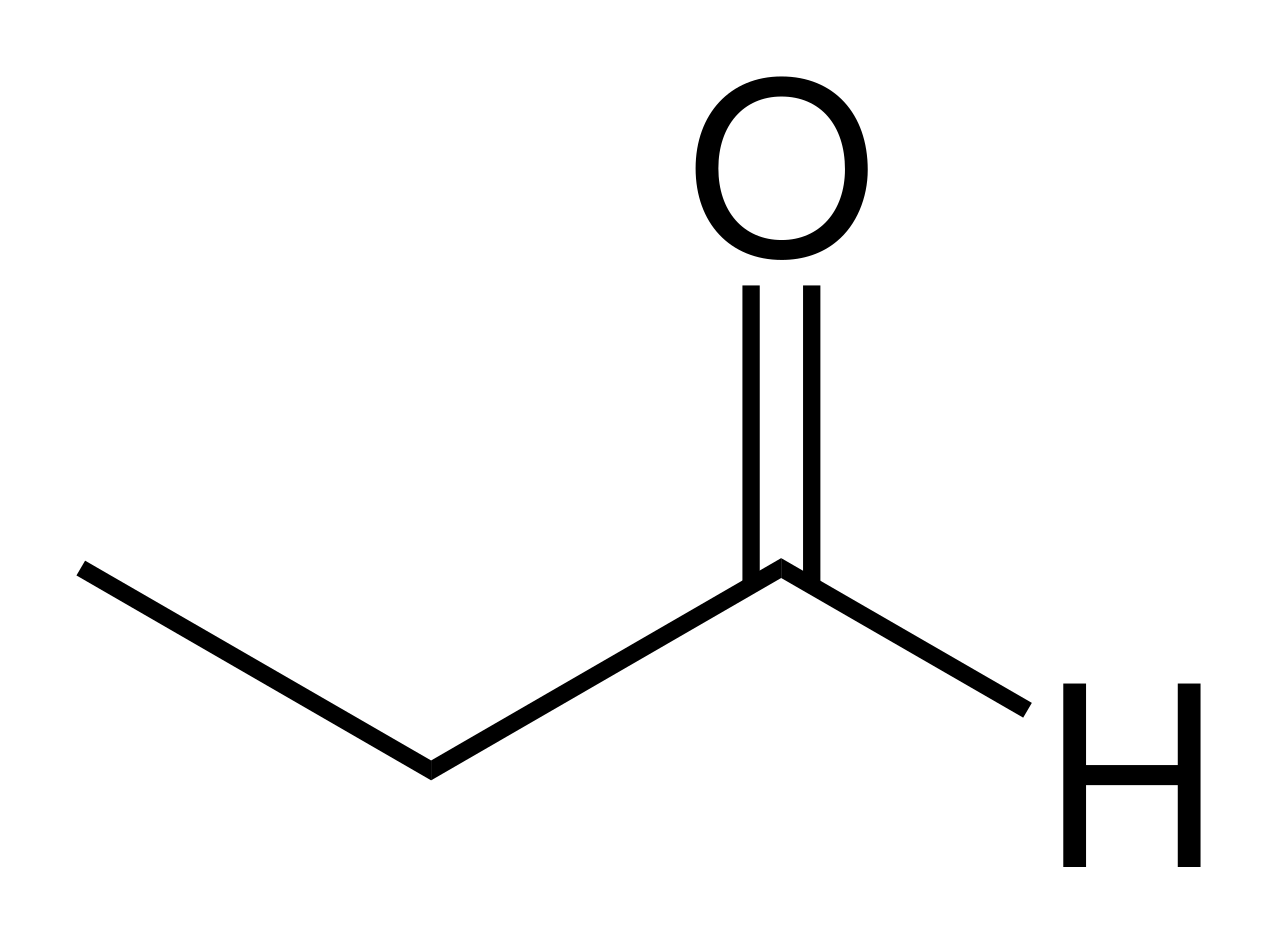
Each point or vertice represents a carbon atoms and each point has hydrogen atoms bonded to it but it is not shown
Functional Group
Reactivity of a compound is determined by the functional group. The functional group is a special atom or a group of atoms etc that uniquely identifies the type of compound. This table will help to summarise them:
| Functional Group | Group | Example |
|---|---|---|
| Alkane | C-C | Ethane |
| Alkene | C=C | Ethene |
| Aryl | -C6H5 | Methyl Benzene |
| Ketone | -C-CO-C- | Propanone |
| Aldehyde | -CHO | Ethanal |
| Ester | C-CO2-C | Methyl ethanoate |
| Alcohol | -OH | Ethanol |
| Carboxylic acid | -COOH | Ethanoic acid |
| Nitriles | -C☰N | Propanenitrile |
| Amine | -NH2 | Methylamine |
Naming Organic compounds
First, you need to identify the functional group present in the compound and check its priority in naming.
This is the order in which you must follow. Starting the highest priority:
1. Carboxylic acids
2. Esters
3. Nitriles
4. Aldehydes
5. Ketones
6. Alcohols
7. C☰C
8. C=C
9. Alkanes or alky groups
This is useful when the compound has many functional groups present in it. Then we need to identify the position of the functional group
By doing this we try to get the lowest value possible. For example, 2-methylbutane is the same as 3-methybutane, but we don't write it like this so we say it is 2-methylbutane because, 3-methybutane is a reflection of 2-methylbutane
Another thing is to make sure you find the longest carbon chain. Then we can call it according to the prefixes below:
| Number of carbon atoms | Prefix |
|---|---|
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
| 6 | Hex |
| 7 | Hept |
| 8 | Oct |
| 9 | Non |
| 10 | Dec |
This is how we start the compound based on the number of carbon atoms in the MAIN chain
The below table will tell the suffix of a compound for each functional group:
| Functional Group | Ends with: |
|---|---|
| Alkane | -ane |
| Alkene | -ene |
| Aryl | Benzene |
| Ketone | -one |
| Aldehyde | -al |
| Ester | Alcohyl Carboxylate |
| Alcohol | -ol |
| Carboxylic acid | -oic acid |
| Nitriles | -nitrile |
| Amine | -amine |
After you identify the main functional group, then you know how to name it. If there are carbon branches or alky groups then we need to know how to name them too and we need to state the position of each functional group:
This is the normal syntax of naming:
5,5 - dimethylhexan-2-ol
If there are two or more of the same ALKY groups we start by saying "di" or "tri" to show the number of alkyl group of each type. Di - means two and Tri - means 3
The 5,5 shows that there are 2 seperate methyl groups connected to the 5th carbon atom
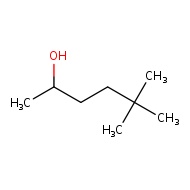
The -2- in the alcohol tells us that the OH group is attached to the second carbon. Understand that alcohols (OH group) has the highest priority in this compound so it will get the lowest number possible. This sets the direction for us to name the position of other functional groups.
Aldehydes and carboxylic acid always has the functional group at the end of the chain and they usually have the highest priority so the group position is 1 but, we can ignore this and just state the functional group. For example:
3-Methylbutanoic acid
Isomerism
There are two types of isomerisms:
The Compound has the same molecular formula but different structures or structural formula
Compounds with the same structural formula and same molecular formula and structures but different arrangement of atoms or group of atoms in space
Each type of Isomerism can be further broken down!
Structural Isomerism
Structural isomerism can arise from 3 different types:
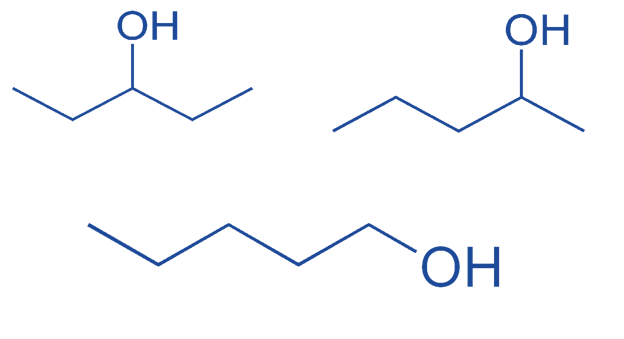
Isomerism which arises from changing the position of the functional group. For example:
Butan-1-ol and butan-2-ol are considered to be positional isomerisms
They arise from the carbon branching. This will have the same number of carbon atoms and the same molecular formula
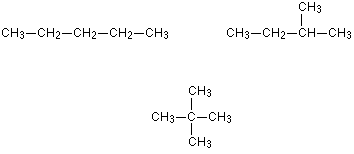
An good example would be 2-methylbutanol and pentanol. The methyl group is attached to the 2nd carbon atom instead of the 1st. Always remember that bending the carbon chain doesn't chain the structure of the MAIN Chain
Personally, I feel the best way to identify structural chain isomers is to draw a skeletal formula as you can see whether the different structures are reflections or not easily
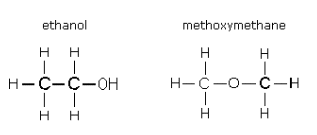
These are compounds which are from two different homologous series or types but happens to have the same molecular fomula. For example, a ketone and aldehyde happens to have the same molecular formula but have different functional groups
Propanone and propanal both has the molecular formula, C3H6O
Stereo- Isomerism
This is when they almost looks identical but, they have different arrangements in space or in other words - 3D space
They are broken down to:
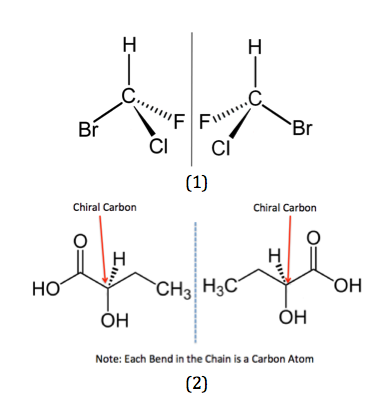
This is when the two isomers are non-superimposable. It means that when they overlap over each other, they are not identical.
More will be discussed down
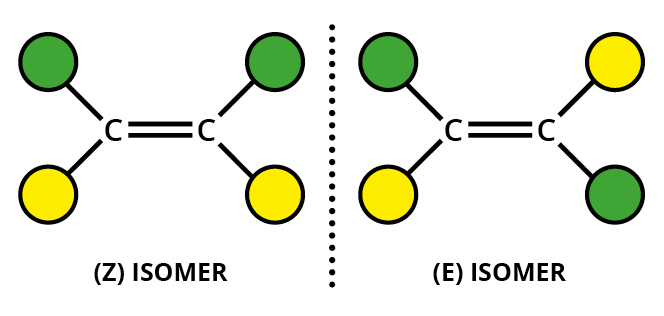
This arises due to the presence of a carbon double bond or triple bond in the compound. Usually carbon atoms can rotate inside a compound if it has a single bond but if there is a double bond there is a restriction in rotation
We will see an example below:
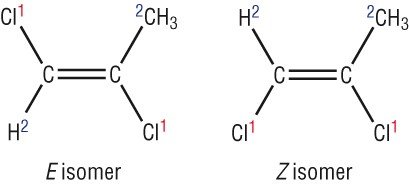
As you can see due to the restriction in free rotation this will result two different isomers
One compound has the chlorine atom aligned or is in the same level to the other chlorine atom. This has the suffix - Cis. The other one has the two atoms diagonally placed so we call this the Trans- isomer
This will result in two different compounds with slightly different reactivities and properties
Here is the Catch
Not all compounds with Carbon double bonds will result in an E-Z isomerism. You need to carefully check whether your assumed Trans or Cis isomerism is not just a rotation or fliping of the other compound. If you can rotate or flip one structure to get the other structure then it is not an E-Z isomerism.

Common examples are Propene and Butene etc where it ends with a =CH2 group as when rotated, it is the same thing
Chiral Center
Before we learn more about optical isomerisms, we need to know what is a chiral center
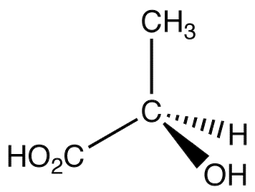
When a carbon atom is bonded to 4 different groups or types of atoms. This makes the structure non symmetrical and non superimposable
In other words, there are 4 different R Groups attached to the carbon atom. The R groups can be a long chain of atoms or it could simply be a hydrogen or a chlorine atom. Always remember that there must be 4 different bonds and so C=C (double bond) can not have a chiral center
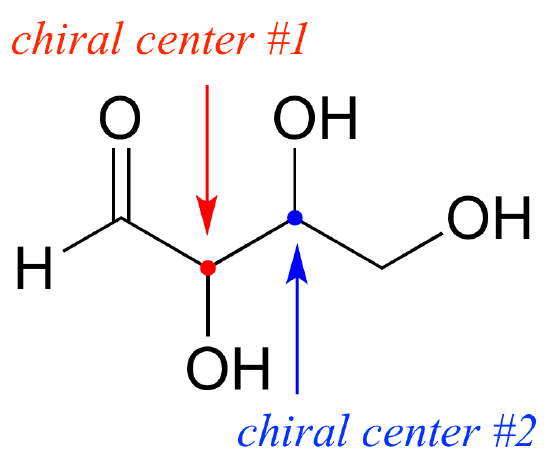
The Chiral center is required for a compound to have an optical isomer. The Other Optical isomer is a reflection / mirror image of the other
This is called an Optical pair and each structure is known as an enantiomer. One is called the left enantiomer and the other one the right enantiomer

They spin in opposite directions and so they polarise light different. They also have different chemical properties
Racemic Mixture
In some reactions, it could produce a racemic mixture. A racemic mixture is a mixture in which it contain equal amounts ( 50% - 50% ) of the left and the right enantiomer. As they spin in opposite reaction they cancel out and have an overall of no spin. It is said to be optically inactive due to this equal cancelling effect